
|
Slide Presentations
National Institutes of Health
Slide Presentation on the Privacy Rule and Research
Slide 25:
| < Previous |
Slide 25 |
Next > |
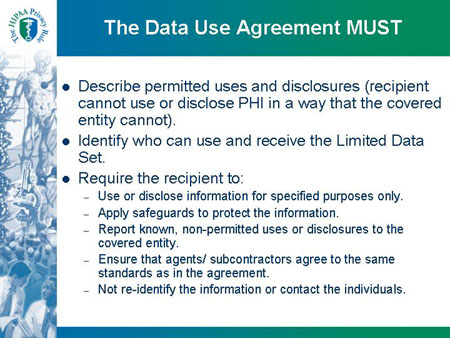
The Data Use Agreement MUST
- Describe permitted uses and disclosures (recipient
cannot use or disclose PHI in a way that the covered
entity cannot).
- Identify who can use and receive the Limited Data
Set.
- Require the recipient to:
- Use or disclose information for specified purposes only.
- Apply safeguards to protect the information.
- Report known, non-permitted uses or disclosures to the
covered entity.
- Ensure that agents/ subcontractors agree to the same
standards as in the agreement.
- Not re-identify the information or contact the individuals.
|
|



